1,000+ of the World's Leading Companies Trust QT9





Centralize Quality Events
- Closed-loop quality event management: Create, track and trend quality event investigations and outcomes in one place.
- Connect quality events: Quality events serve as your central hub to quality issues that can create corrective actions, nonconforming products, customer feedback, and even deviations.
- Eliminate data silos: Work together in one system for accurate traceability and compliance.
- Manage custom fields: Create unlimited user defined fields and sections to save your best practices for the future.
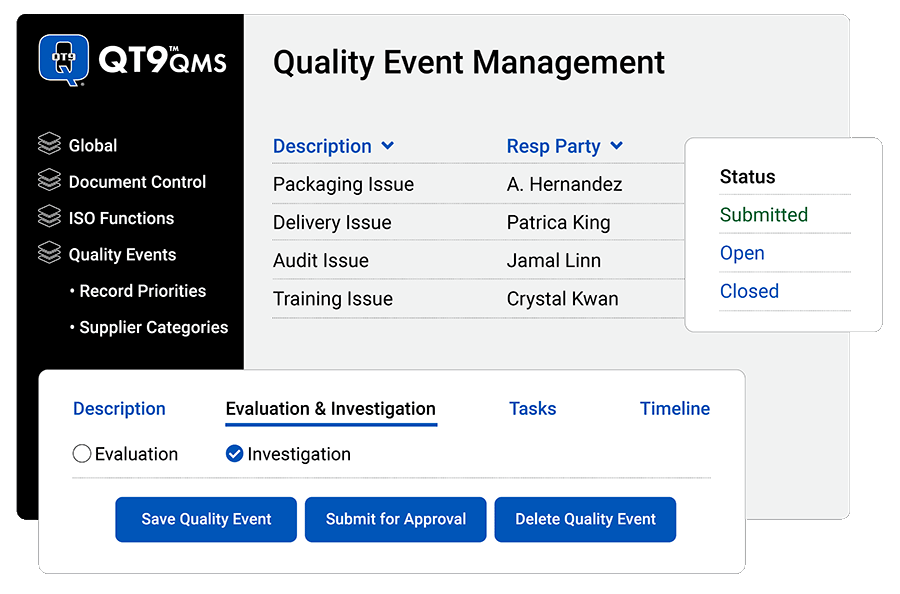
Capture End-to-End Quality Event Management
- Record any event affecting your quality system: Enable your team to investigate, delegate tasks, attach files, tag types and categories for trend analysis, and link to other modules in QT9 QMS.
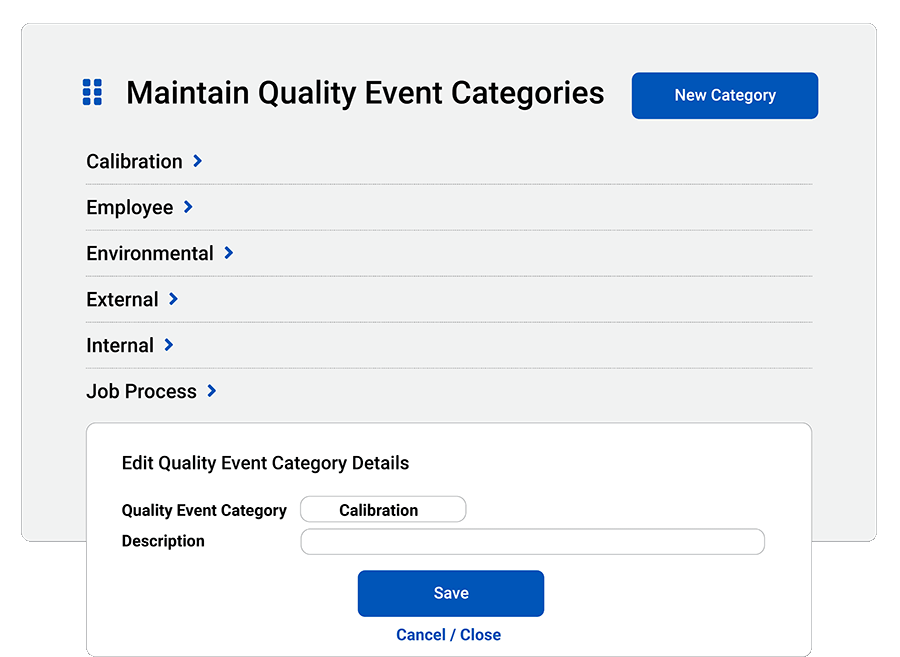
- Assign categories: Organize quality events with your own types, categories and dispositions.
- Manage workflows: Route action items to the right person with automated reminders.
- Standardize quality event processes: Consistently track quality events the same way to ensure no information gets missed.
Track and Trend Quality Events
- Real-time reporting: Keep pace with trends in quality events for better decision-making.
- See quality events your way: Make it easy to see the most relevant information for your team by creating multiple custom views within quality events.
- Stay on track: Reduce delays with automation efficiencies, such as email alerts and electronic signature approvals.
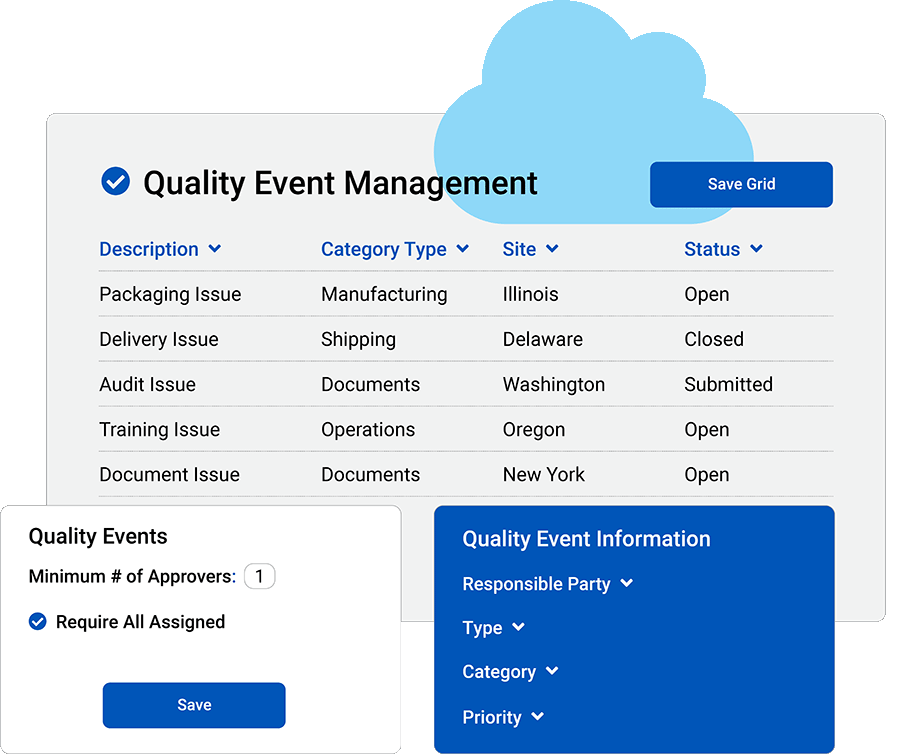
Integrate your quality events.
Create a shared hub for collaboration.
Improve Collaboration
Link quality events to customers, suppliers, processes and products.
Manage Tasks
Assign responsibilities, set deadlines and track progress.
Responsible Parties
Pre-assign approvers and create responsible parties.
Improve Traceability
See a timeline of every action along with lot and serial traceability.
Get Real-Time Analytics
Make informed decisions with real-time dashboards. Create your own custom views.
Ensure Compliance
Show proof of proper handling, supporting FDA Current Good Manufacturing Practices (cGMPs).
Initiate investigations
Launch & explore evaluations & investigations. Assign dispositions & track verification notes.
Link Files
Tackle quality events together by sharing files attachments to keep everyone on the same page.
See What Quality Leaders Say About QT9
"All of our records are now in one location"
QT9 has streamlined our internal auditing process, preparation for regulatory audits, document control and approval, and organizational training. We have been able to reduce our active documents by 30% as unnecessary/redundant documents and procedures have been made more visible through the document review process.
Reviewed on Capterra
![]()
Jennifer B.
Medical Device Industry
Small-Business (11-50 emp.)
Read the Review
"Looking forward to using more modules"
The document control feature has improved our ability to find documents and some records when requested for audit or project purposes. Training is much easier to manage. I like being able to link equipment and gages to system documentation in the Document list. The customer service has always been helpful and responds quickly.
Reviewed on Capterra![]()
Bill C.
Medical Device Industry
Mid-Market (51-200 emp.)
Read the Review
"Excellent support, traceability and ease of use."
We are a medical device company and myself, the FDA & ISO auditors liked the traceability, control of documents and everything was there at our fingertips from Management review to Documents to CAR's. QT9 is always responsive to our questions and resolving issues. For the cost, the flexibility, electronic signature and traceability is worth trying out the system.
Reviewed on Software Advice![]()
Reba D.
Medical Device Industry
Director of QA/RA
Read the Review
Connect All the Tools You Use for Quality Events
QT9 QMS enables you to integrate multiple quality process with product design.
Proven Impact Across Organizations
Explore ways your team can use QT9 QMS to work efficiently and drive results.
Frequently Asked Questions: Quality Events
What is a quality event?
A quality event is part of a quality management system used to identify any departure from an expected behavior that could effect quality. This could be anything from an incorrect temperature recording to a customer complaint.
What is the quality event process?
Every quality event is different, but having a standardized process to follow helps ensure no task is overlooked. The general framework to address a quality event involves collecting basic information about the incident, assessing level of risk, initiating an investigation if so warranted, and documenting each step for compliance and future trend analysis. The QT9 QMS Quality Events Module standardizes the process and automatically documents processes for easier tracking and traceability.
Dynamic Dashboard Grids
Quickly view documents from the QT9 dashboard, and easily personalize the view with the data that is most important to you.
Traceability & Transparency
QT9™ QMS includes document control procedures that ensure documents get reviewed and approved timely.
Central Hub for Quality Issues
Create corrective actions, nonconforming products, customer feedback, and even deviations.





