
Introduction
What is change control software?
What are the benefits of using change control software?
What are ISO and FDA change control requirements?
What types of changes benefit from change control software?
What is the change control process?
Navigating Change Using Change Control Software
May 14, 2024
Those in the Quality world know from experience the truth in the saying that “the only constant is change.” Policies change, procedures change, regulations and a host of other variables will change. As quality professionals, we need to manage each and every instance. Uncontrolled changes can have disastrous consequences, leading to product defects, compliance breaches and, ultimately, a damaged reputation.
Change control software is the solution. Change control software helps organizations streamline and manage change as part of their quality management system (QMS), integrating with key quality processes, such as document control and nonconforming products. This article will explore the role of change control software in quality management and equip you with the knowledge to make informed decision when choosing a change control platform.
What is change control software?
Change control software automates change management tasks, makes them centrally accessible and creates a digital record of each step in the process. The software provides a structured framework for initiating, evaluating, approving, implementing and tracking product or process modifications, while ensuring continued compliance with regulatory standards and quality protocols.
Change control is an integral part of the quality management process and is required by national and international manufacturing and regulatory standards. Any modern eQMS should include a change control software application that provides manufacturers with an integrated system for initiating, managing and tracking the change process. The goal of change control software in an eQMS is to not only track and document modifications, but also set the stage for a thorough evaluation of risks, impact and outcomes.
What are the benefits of using change control software?
When changes are necessary in manufacturing, controlling the change is imperative to ensuring quality, especially in highly regulated industries, such as pharmaceuticals and other life sciences.
Mitigate Risk
One change can have a ripple effect, the results of which are often not immediately evident. By implementing a structured change control process, organizations minimize the risk of introducing errors and nonconformities that can have detrimental consequences.
Improve Efficiency and Productivity
Automated change workflows and centralized information make it easier for employees to move through the change process, freeing them up to focus on core objectives. Being prepared for change with a structured system minimizes the impact of change on physical resources as well.
Simplify Traceability
Change control software and its integration with other quality processes gives your business total insight into your manufacturing and change control efforts through robust data capture and documentation. This helps ensure quality and compliance, ultimately improving customer satisfaction.
Access Real-Time Data
Change control software puts at your fingertips valuable insights into your business’ production and manufacturing trends, enabling you to make data-driven decisions and to identify areas for improvement.
Ensure Regulatory Compliance
Having a built-in, systematic procedure for change control satisfies regulatory requirements that call for change management plans to be in place. Change documentation is automatic and easily accessible via the software.
What are ISO and FDA change control requirements?
The International Organization for Standardization (ISO), Good Manufacturing Practice (GMP), and the U.S. Food and Drug Administration (FDA) all point to the importance of planning for and managing change in their regulatory standards.
ISO 9001 requires an organization to have plans in place to manage the change process, review the consequences of unintended changes and prevent or mitigate any adverse effects. GMP guidelines state, “Significant amendments to the manufacturing process, including any change in equipment or materials, which may affect product quality and/or the reproducibility of the process should be validated.”
FDA 21 CFR Parts 210, 211 and 820 require manufacturers to establish and maintain procedures for the identification, documentation, validation, verification, review, and approval of design and manufacturing changes. Changes to documents require review and approval by qualified individuals and communication of changes to stakeholders in a timely manner.
What types of changes benefit from change control software?
Any quality event can trigger the change control process. Some of the most common types of changes include:
- Customer feedback/complaint
- Audit finding
- Corrective action (CAPA)
- Policy or standard change
- New equipment
- Process change
- Document revision
Once the need for change is identified, it is documented and the change process initiated.
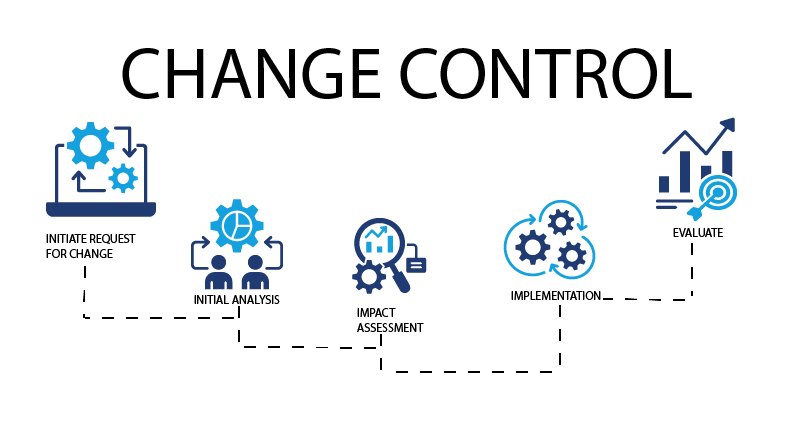
What is the change control process?
Change control software automates and streamlines the change control process, replacing disjointed manual methods, which can create bottlenecks and are more easily subject to error.
In general, change control software encompasses the following steps:
- Change request submission – Once the need for change is identified, a change request is initiated via the software, generally using a templated form or dashboard. This helps to consistently detail proposed modifications and the reason for them.
- Initial analysis – The requested change is evaluated to ensure all details are in place, including resources needed and validity of proposed implementation plan. Based on this information, the request is routed to the appropriate stakeholders to determine next steps.
- Impact assessment – Once all details are in place, the change request is analyzed by an appropriate guiding authority and approved or denied. If approved, the change is communicated to stakeholders impacted by the change and preparations are made for implementation.
- Implementation – Approved changes are implemented in a systematic manner, ensuring that any modifications are closely documented. Each required adjustment must be analyzed and verified. After the change request receives approval for closure, stakeholders are trained on any new processes or procedures.
- Evaluate effectiveness – After the change is implemented, it must be monitored to ensure outcomes are as predicted and needed. Any deviations from expected results are identified and addressed.
What are the key features of change control software?
When considering change control software, several features are instrumental in ensuring the most comprehensive control.
Standardized Workflows
By having pre-defined, easily accessible procedures in place for change control, employees can better ensure that nothing is missed during the change process. Documents are routed to and approved by the appropriate people, then logged accordingly. Documents are located in one application, making them easily retrievable for traceability and other compliance purposes.
User-Defined Fields
Not all businesses will have the same change control needs, so being able to make at least enough customization to address your needs is important. A good mix of templates and the ability to adjust for user needs offers a tailored, simplified experience.
Automated Integrations
Change control software that seamlessly integrates with other quality processes, such as corrective and preventive actions (CAPA) and document control, improves the overall efficiency and excellence of your quality systems. Communicating changes, initiating training and maintaining compliance records are just a few of the tasks that are simplified with change control integrations.
Robust Data Analysis
Integrated quality processes give you more robust data and foster the ability to gain valuable insights into change trends and identify areas for improvement. QT9’s Business Intelligence tool provides advanced reporting capabilities that allow you to visualize your data in the most applicable manner.
Cloud Based
A change control software solution that is based in the cloud can be accessed anytime, from any device, making it easier to collaborate with offsite stakeholders. In addition, cloud-based software is generally more agile, with the ability to more easily deploy and make updates.
Finding the best change control software provider
Change control software is a powerful tool that empowers organizations to manage change effectively within their quality management systems. By carefully considering your specific needs and choosing the right software solution, you can transform change from a chaotic process to a controlled and valuable driver of continuous improvement in your organization.
The need for change control software will vary depending on your industry. QT9 QMS offers a change control solution that offers out-of-the-box benefits with the ability to be tailored to a business’ specific needs.
QT9 QMS provides 25-plus modules standard, including document control, CAPA management, audit management and supplier management. It is versatile for companies of any size and is consistently one of the top-rated quality management systems available.
Related Content


