Pharmaceutical Software
Made Easy
QT9™ QMS and QT9™ ERP Cloud can automate your quality and operational processes. Centrally manage ISO & FDA compliance with one connected, simple, seamless platform.
Schedule a Free Demo and Start a Free Trial Today:
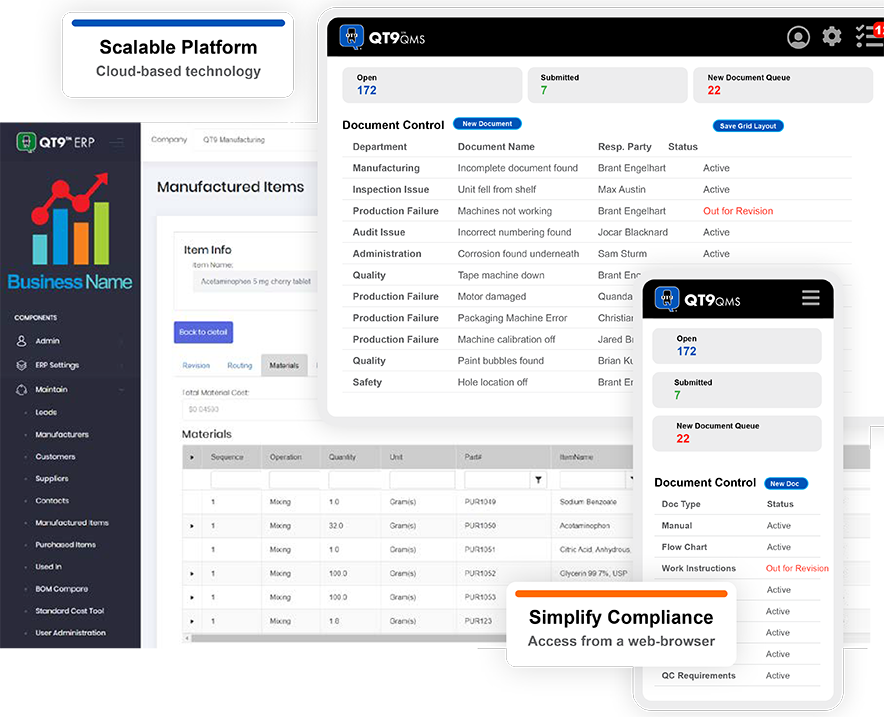
All-In-One Pharmaceutical Software
Simplify FDA and ISO compliance with QT9's intuitive cloud-based platform.
FDA 21 CFR Part 11
Electronic Signatures
FDA Compliance
FDA 21 CFR Part 210/211
Pharmaceutical Companies
FDA Compliance
FDA Compliance Software
ISO 9001
Quality Management System
ISO Compliance
ISO 14001
Environmental Management
ISO 17025
Laboratory Testing & Calibration
QT9 supports Current Good Manufacturing Practice (cGMP) regulations as well.
QT9™ ERP Automates Electronic Batch Records
One of the powerful features of QT9™ ERP Web is its ability to generate single-level and multi-level Electronic Batch Records (EBR) and Master Batch Records (MBR) for the pharmaceutical industry. These features plus our FREE validation report make QT9 ERP Web a must have for any pharmaceutical manufacturer.
Master Batch Record
Utilizing your Bill of Materials, QT9 QMS documents, QT9 QMS inspection plans, and file attachments, QT9 ERP Web can deliver a “one click” MBR at your fingertips. No paper needed. No separate folder structures. MBRs are tied to any revision of your manufactured item’s Bill of Materials.
Electronic Batch Record
Print off your Electronic Batch Records from your completed and approved jobs. EBRs in QT9 ERP Web can be multi-level, utilize QT9 QMS inspection records, labels, job file attachments, and custom job print outs. No paper or manual compilation of Batch Records is needed in QT9 ERP Web.
Compliance Portal
The QT9 ERP Web compliance portal is a separate web portal for users to guide FDA auditors to EBR and MBR records with printout capabilities without exposing auditors to the main ERP Web interface. The compliance portal also has the ability to allow approvals on jobs to push completed inventory into an available status.
QT9 ERP handles validation for life sciences, medical device manufacturers, pharma and biotech companies.
Connect your pharmaceutical operations
Your pain? We understand. This is why we do what we do, and can provide you with an experience like no other.
MBR and EBR Capability
- One-click print MBR and EBR
- Compliance Portal for auditors
- Eliminate paper processes
Streamline Operations
- Bill of Material revision control
- Visual production scheduling
- Shop floor management
Automate Compliance
- 21 CFR Part 11 Compliant
- Software Validation Included
- Support cGMP & FDA standards
Real-Time Reporting
- Filter, summarize & export data
- Performance dashboards
- Custom reporting
Control Inventory
- Expiring inventory alerts
- Lot & serial traceability
- Barcode inventory for accuracy
Purchasing & Planning
- Reorder points & lead times
- MRP & forecasting capability
- PO inspections and approvals

Simplify pharmaceutical traceability
Streamline how pharmaceutical manufacturers share, access and collaborate across roles, departments and locations. QT9 ERP provides a central place to know who worked on what, when, with FDA 21 CFR Part 11 electronic signature approvals and transactional audit trails.
Digitize pharmaceutical quality workflows
Don't waste time on time-consuming, redundant tasks. No matter where your team works, QT9 ERP can automate it for you to deliver high-quality, safe products consistent with Good Manufacturing Practices (GMP). Our integration into industry leading QMS software (QT9 Quality Management Software) gives you one single platform for your pharmaceutical manufacturing business.


Protect Against Recalls
QT9 ERP helps you minimize risk by enabling you to control every revision of your Bill of Materials, so you know who ordered what, when it was manufactured & exactly what materials went into it. Find recall information in seconds rather than days.
Drive innovation with continuous improvement
Innovate with an adaptable platform
Modernize your quality management system with mobile capabilities and global administrative tools that make it easy to add user-defined fields and customizable reports on a flexible, scalable and secure platform.
Support continuous business growth
Digitally transform your organization by unifying information and compliance processes with connected modules. Expand at your pace with unlimited file attachments and unlimited data space. FDA 21 CFR Part 11 compliant electronic approvals simplify approval processes.
Mitigate potential risks
Automate risk management with uniform methods that make it simple to conduct risk investigations efficiently. Create risk assessments from over half the modules in the QT9 QMS.
Increase scalability and agility
Easily adjust QT9 QMS beyond your existing workload while keeping everything contained in one place. The QT9 QMS is a highly adaptive system with flexible deployment options that allow you to adopt for future growth.

Advance your involvement of people
Empower employees to be more productive
Enable employees to be more effective (or employees do their job more efficiently with tasks, reminders and To-Do lists to make sure everything gets done by the right person at the right time with greater flexibility.
Establish accountability and prevent delays
Assign documents to your colleagues or customers for approval. Minimize delays with automated email alerts & reminders with hotlinks when important tasks are coming due.
Connect and collaborate online
Enable your team to come together anywhere. Share documents, escalate corrective actions or attach files to any task with the click of the button. Web portals for customers, suppliers and employees are included.
Work on-the-go anytime and anywhere
Manage your quality management software from anywhere with our web-based interface. Review documentation and tasks no matter where you are to prevent delays and improve response times.
Optimize your process approach
Boost productivity
Automate routine tasks by implementing quality management best practices quickly across your organization. Accelerate product delivery by controlling every process to get more done on time and under budget.
Automate workflow
Save time and eliminate manual processes to help achieve operational excellence. QT9 QMS's fully integrated modules allow data to be inherited from one process to the next.
Ensure quality standards are being met
Enable paperless compliance for your regulatory standards including: FDA, ISO 9001, ISO 13485, ISO 17025, AS9100, IATF 16949, HACCP, SQF, etc.
Simplify compliance
Manage your compliance with ease by integrating quality and compliance management processes, complaint handling, document control, and training management, and more, on a single platform.

Synchronize processes for mutually beneficial supplier relationships
Connect with suppliers anywhere
Connect data and quality management processes between your suppliers and distributors with the supplier web portal included with the QT9 QMS. Stay in touch with partners where they are for greater flexibility.
Keep quality processes consistent
Automatically send corrective actions, nonconformance's and evaluations to your suppliers as well as share documents to reduce communication gaps and ensure the right actions are taken within your quality management software.
Connect and collaborate online
Enable your team to come together anywhere. Share documents, escalate corrective actions or attach files to any task with the click of the button. Web portals for customers, suppliers and employees are included.
Reduce quality gaps
Establish comprehensive quality management measures with QT9 QMS. Email corrective actions or nonconformances directly to suppliers to move action items forward and minimize disruptions.
Prevent delays
Speed up response times by eliminating unnecessary printed materials and digitally connecting suppliers directly to your data. Minimize disruptions to stay on time and under budget.
Elevate your customer focus
Deliver exceptional customer experiences
Maintain happy customers by preventing service and support issues with quality management software. Transform your customer interactions from reactive bursts to proactive strategies with real-time insights.
Build customer loyalty
Build positive relationships to earn repeat business with the customer web portal for responding to surveys, approving documents and approving engineering change requests.
Streamline feedback and complaint processes
Resolve customer issues quickly with a built-in customer feedback module that tracks customer feedback history based on your feedback categories within the QT9 QMS software.
Easily engage with your customers
Enhance customer communication with a customer web portal that links corrective actions, nonconforming products, engineering change requests, documents and surveys directly to customers in your quality management system.

Leverage your data for a factual approach to decision making
Easily create dynamic dashboards
Create your own personalized dashboard grids, so you can quickly get a picture of your quality management results and regulatory compliance at a glance. Intuitively drill down and filter data the way you want, and export it to Excel for further analysis.
Keep quality processes consistent
Automatically send corrective actions, nonconformance's and evaluations to your suppliers as well as share documents to reduce communication gaps and ensure the right actions are taken within your quality management software.
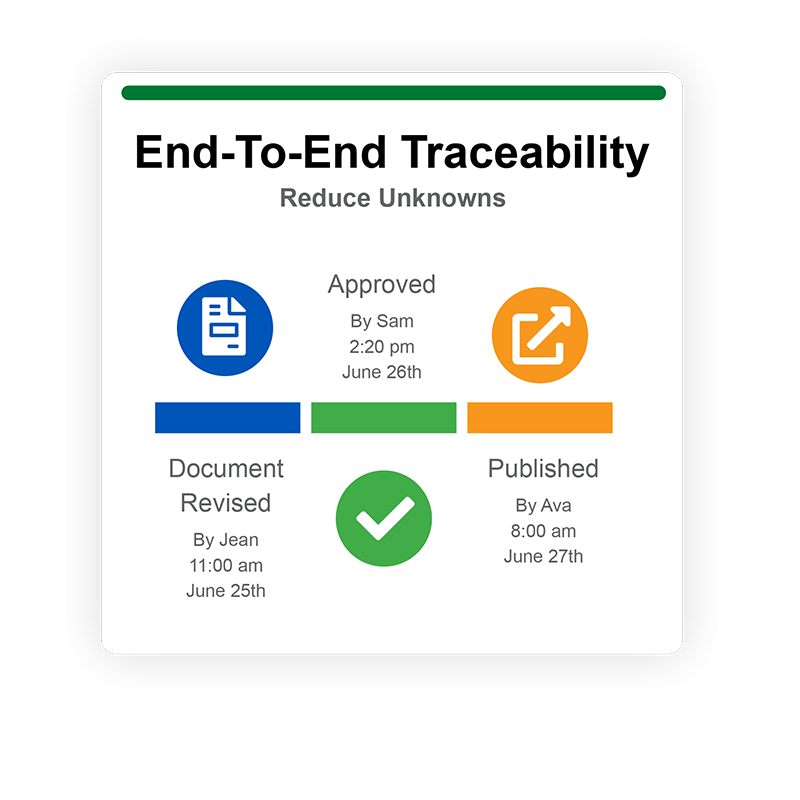
Experience total traceability
Track every single action in QT9’s timeline to reduce unknowns with a full audit trail that shows the history of all actions. Make informed decisions with date/time/name stamped entries to show which user made the changes and when the changes were made.
Turn insights into action
Improve compliance by gaining better visibility of what works and what doesn't from your quality management software. Effectively assign resources by pinpointing critical issues and assessing future trends.
Make calculated decisions
Anticipate and solve problems immediately by reducing the time, cost and complexity of identifying specific inefficiencies and quality management gaps. Access insightful data to get the big picture.
Unify your system approach to management
Integrate seamlessly with existing systems
Dynamically populate modules with data inherited from one process to the next within the QT9 QMS. Centralize all your quality and compliance management processes with unlimited file attachments and interconnected modules.
Synchronize data across multiple sites
Connect data between multiple locations into one single source of truth. Manage global initiatives from anywhere to be more productive and consistent in quality management.
Simplify ERP integration
Seamlessly transfer data back and forth between the QT9 QMS and QT9 ERP (and other ERP systems) to automate business functions. Modernize your production and operations for faster, easier and more efficient results.
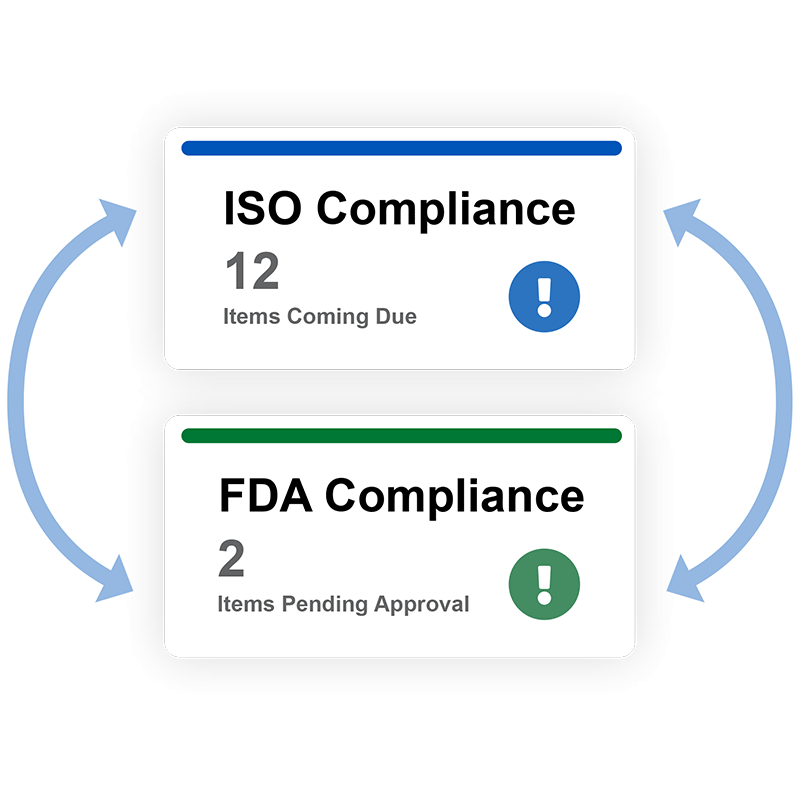
Automate ISO and FDA compliance
Link all of your quality compliance information together and replace your spreadsheets. Wrapping your arms around your data is faster and less costly when it is centrally managed in the QT9 QMS.
Collaborate with leadership
Establish unity to drive results
Centralize quality management processes for better ISO and FDA compliance. Easily keep track of compliance activities and audits on a common platform to ensure consistency across your organization.
Strategize with precision
Turn insights into action with real-time analytics on corrective actions, audits and risk assessments. Proactively identify trends and address concerns to create better quality management processes while reducing costs.
Create a global infrastructure
Manage global company-wide initiatives on a single platform for greater control. Align your company-wide quality management procedures with industry best practices.
Modernize quality management
Evolve at a faster pace by maximizing the efficiencies of your existing resources. Automate manual processes and eliminate paper forms to maximize your ROI with quality management software.
QT9 enables pharmaceutical companies to adapt quickly.
Multi-Site Architecture
Connect data from multiple locations.
Cloud-Based Platform
Scale quickly with a web subscription.
All Modules Included
Unlock capabilities as needed.